SMMRT™ Pipeline
Our small molecule combinations can be adapted to work synergistically on biofilms and microbiomes for positive outcomes.
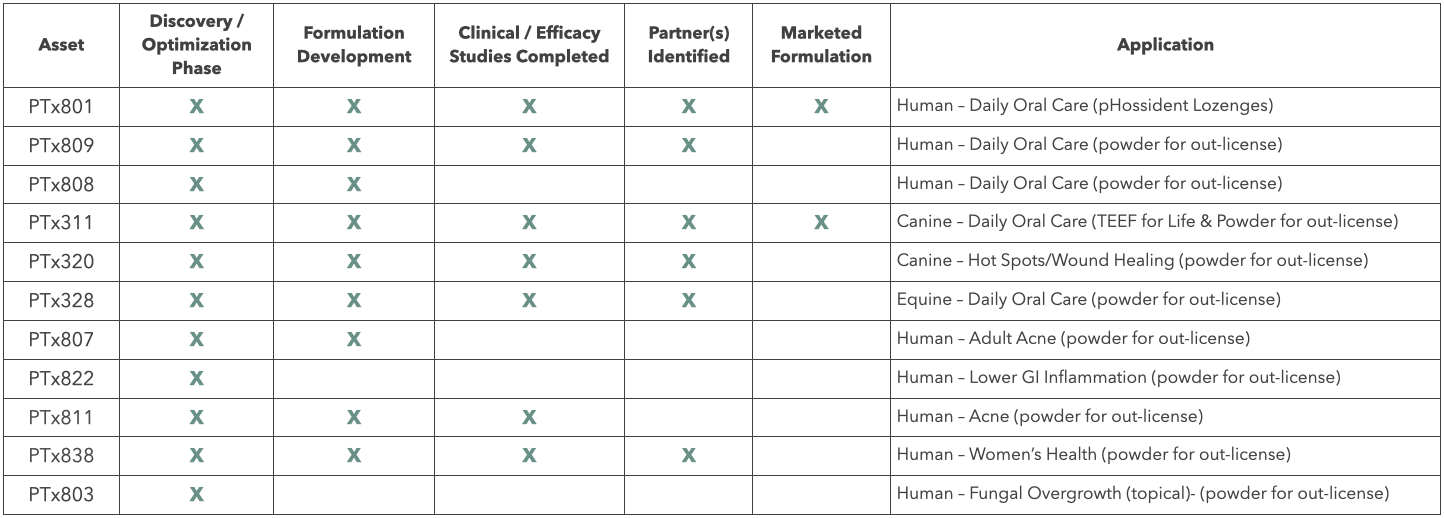
Primal Therapies's IP estate contains many different novel compositions that solve problems across various industries. Not listed below are industrial, agricultural, and environmental applications that solve for AMR, Infection control, and dysbiotic/imbalanced microbomes).
ALL assets in our pipeline are new drug indications or Generally Recognized as Safe (GRAS) by the FDA.
AMR Applications - PTx811
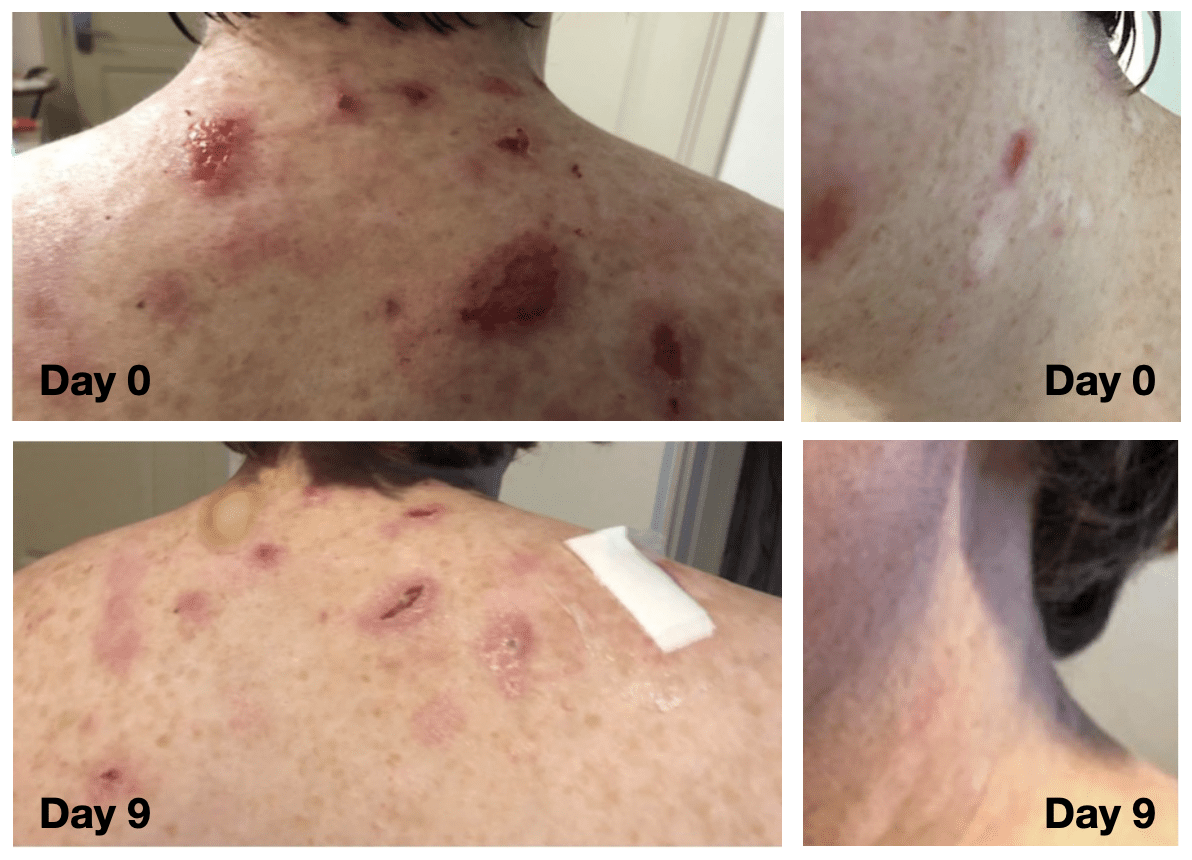
Confirmed Multi-drug resistant Staph (MRSA) skin and soft tissue infection - refractory to all approved antibiotics. Daily topical administration of PTx811 for 14 days, resolution and no reinfection observed after treatment.
Disinfection Applications - PTx801
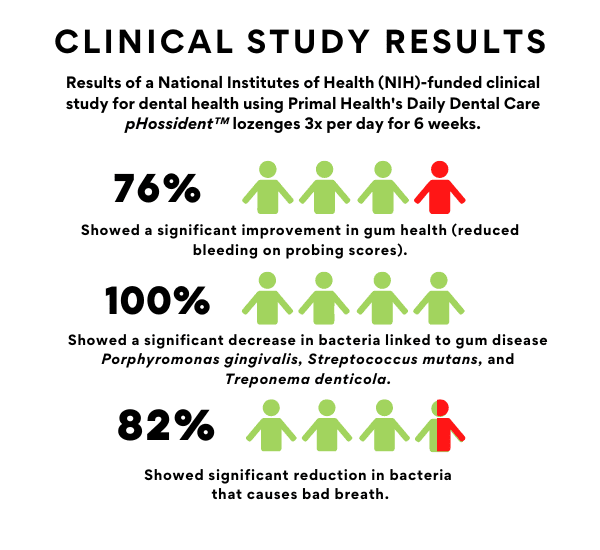
In a placebo-controlled double blinded study (National Institutes of Health) on gingivitis patients, PTx800 demonstrated superior performance in inhibiting dental pathgens, AMR, and clinical disease scores compared to placebo.
Microbiome Applications - PTx838
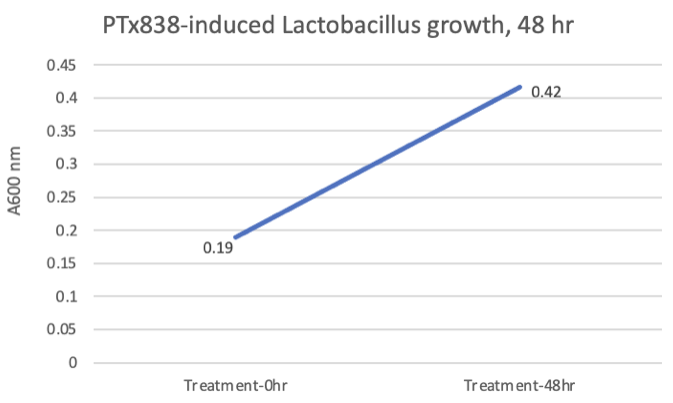
Bacterial vaginosis (BV) and yeast infections are common infections in women that affect over 140MM women annually and can lead to pregnancy complications and increased STI risk. Decreased Lactobacillus sp. result in susceptibility to these infections. We've conducted feasibility studies demonstrating the ability to correct dysbiotic vaginal microbiomes within 48 hours.
Ingredients in this study include a proprietary combination of plant-based prebiotics and a postbiotic.